Diafiltration by cross-flow filtration
Diafiltration is a technique used on liquids to separate large molecules from smaller soluble molecules. It is commonly used to increase the purity of a food or chemical product, or as a buffer exchange in pharmaceutical operations. Nanofiltration (NF), Ultrafiltration (UF), or Microfiltration (MF) membranes may be used. The membrane type depends on the composition of the materials to be separated. For example: NF is commonly used for removing a salt such as sodium chloride from a reactive dye, or an optical brightener. As another example, UF is used for separating lactose from whey protein in the dairy industry.
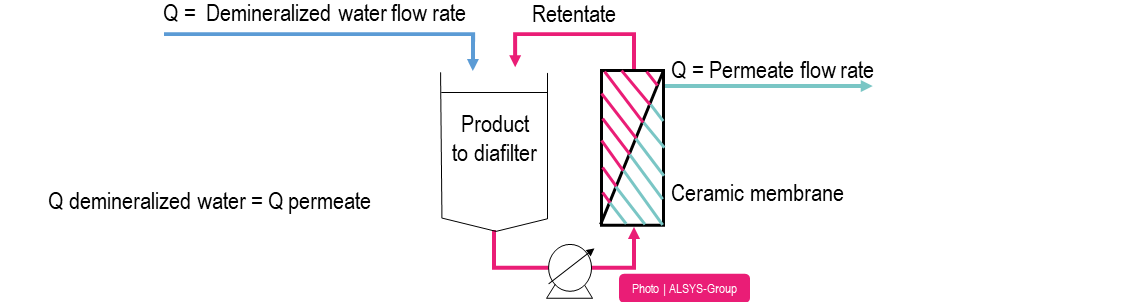
Schematic diagram of the diafiltration process
Diafiltration is performed by adding fresh water to the process fluid during the membrane system operation. The same amount of water added to the solution is removed as permeate. The permeate/water will carry with it soluble components of the process fluid that are not retained by the membrane. When diafiltration has concluded, the concentration of solutes in the process fluid are reduced compared to what would be possible without use of diafiltration.
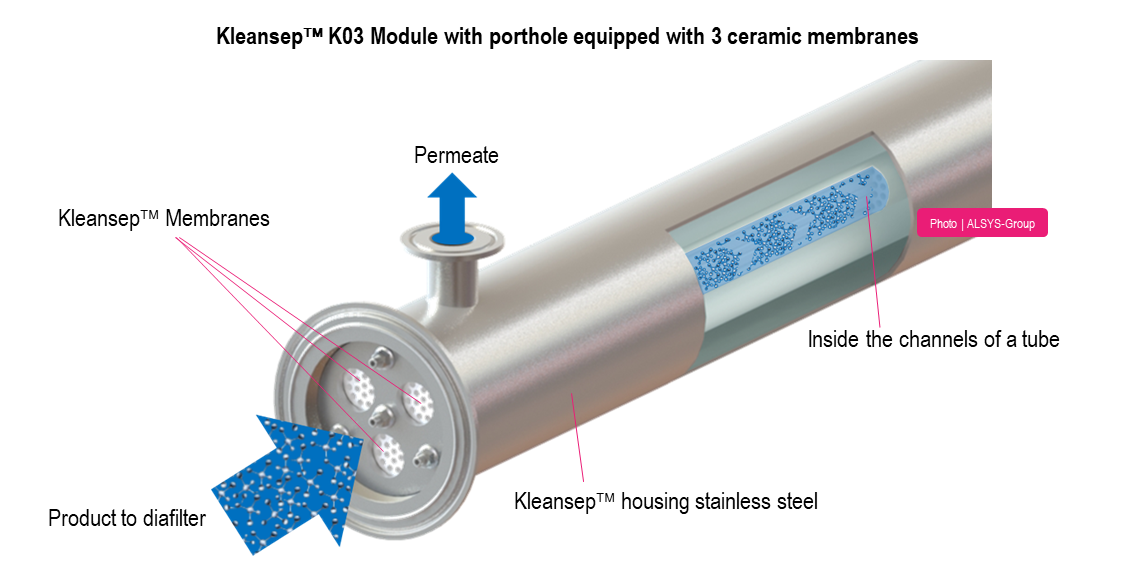
Filtration module
Result of the diafiltration
Left: Product to diafilter (concentrate)
Right: Permeate after diafiltration (with molecules from concentrate)
Diafiltration may be performed in batch or continuous mode. The volume of water needed depends on the initial and desired final concentration of solutes in the feed, the molecular weight of the solutes, and the membrane retention characteristics.
Purified water can be recovered from permeate/diafiltration water with use of NF or Reverse Osmosis (RO).
If you would like more information or assistance, please contact: orelis@alsys-group.com